How to choose the right TENS and EMS equipment for your market?
Choosing the right products for your business feels like a gamble. With so many TENS and EMS devices available, picking the wrong one means wasted inventory and unhappy customers.
To choose the right TENS/EMS equipment, you must first match the device's function (pain relief vs. muscle training) to your target customer. Then, you need to rigorously vet your supplier's qualifications and ensure every product meets the strict regulatory standards of your market.
I've built my entire career in the personal care and health appliance industry. I’ve been on the factory floor and in the boardroom, and I’ve helped countless partners navigate this exact challenge. The difference between a best-selling product and a warehouse failure often comes down to these initial choices. Getting it right isn't about luck; it's about asking the right questions from the start. Let's break down what you need to know.
What Are the Differences Between TENS and EMS? A Selection Guide for Retail and Medical Channels?
A customer asks for a "muscle device," but do they need pain relief or fitness support? Selling them the wrong one leads to returns and damages your credibility. A clear understanding is essential.
TENS (Transcutaneous Electrical Nerve Stimulation) is for pain relief; it blocks pain signals. EMS (Electrical Muscle Stimulation) is for muscle training and rehabilitation; it causes muscles to contract. Your choice depends entirely on your customers' primary need.
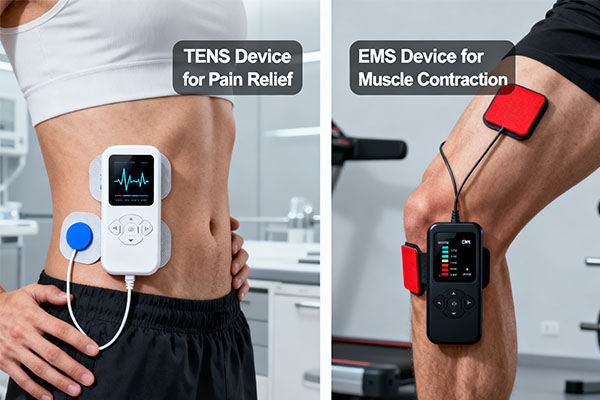
This is the first and most fundamental decision you’ll make. When I work with a new distributor or retail buyer, we always start here. You are not just selling a device; you are selling a solution. A customer with chronic back pain needs a TENS unit. A physical therapist or fitness club needs an EMS unit for muscle re-education and recovery. As a buyer, you must tailor your inventory to your specific sales channel. A general retail store will benefit from user-friendly, well-designed combination units that offer both functions. A specialized medical supply channel, however, requires devices with professional-grade, precise controls and clinical evidence to back up their claims.
Channel-Specific Selection Guide:
| Channel | Primary Focus | Key Features to Look For |
|---|---|---|
| Retail & E-commerce | Ease of Use, Broad Appeal | Intuitive interface, clear pre-set programs, attractive design, combo TENS/EMS functionality. |
| Medical & Wellness | Efficacy, Precision | Separate, precise controls for all parameters, clinical documentation, robust construction for heavy use. |
How Should B-End Procurement Evaluate the Qualifications and Reliability of TENS/EMS Suppliers?
You found a supplier with a tempting low price. But late deliveries, inconsistent quality, and zero support are now costing you more in the long run. A cheap quote can be a very expensive mistake.
To evaluate a supplier, you must look beyond the price. Verify their quality management system (ISO 13485 is ideal), assess their true production capacity through factory audits, and test the responsiveness of their after-sales support.

I learned early in my career that the right partner is worth far more than the lowest price. A reliable supplier is the foundation of a stable business. When you're considering a new factory, here are the non-negotiables you must investigate. First is their quality system. For a medical device like TENS/EMS, you should look for ISO 13485 certification. This isn't just any quality standard; it's specifically for medical device manufacturing and it proves they have rigorous processes. Second, understand their real production capacity. Don't just take their word for it. If possible, visit the factory or get a third-party audit. Can they actually handle your order volume without cutting corners? Finally, check their after-sales support. Before placing a large order, see how they handle sample requests or technical questions. A slow or unhelpful response is a huge red flag for the future.
What Regulations and Standards Must TENS/EMS Devices Meet When Exporting to European and American Markets?
You're ready to expand into profitable Western markets. But a single compliance oversight can get your entire shipment blocked at the border, leading to a financial and logistical nightmare.
For export to the European Union, TENS/EMS devices must comply with the Medical Device Regulation (MDR) and have a valid CE mark. For the United States, they typically require FDA 510(k) clearance before they can be legally marketed.

Navigating international regulations is one of the biggest hurdles for distributors. These are not just suggestions; they are the law. Any supplier you work with must be able to provide proof of compliance. In simple terms, for the EU, the CE mark on a medical device like a TENS unit signifies that it has been thoroughly reviewed for safety and performance under the MDR. For the US market, the Food and Drug Administration (FDA) is in charge. Most TENS/EMS devices fall under a category that requires a "510(k) premarket notification." This means the manufacturer has proven to the FDA that their device is substantially equivalent in safety and effectiveness to a device that is already legally on the market. Asking for and verifying these documents is one of the most important due diligence steps you can take. A supplier who cannot provide them is not a viable partner for these markets.
What Does CE Certification Mean for TENS and EMS Devices? Process and Common Misconceptions?
Many people see a CE logo and assume it's just a basic quality stamp. But you've heard rumors that not all CE marks are the same, and now you're worried about compliance risk.
For a medical device like TENS/EMS, a legitimate CE mark is not a self-declaration. It is a formal certification awarded by a third-party "Notified Body" after a rigorous audit of the device's safety, performance, materials, and clinical evaluation.

This is a critical point that causes a lot of confusion. There are different paths to CE marking depending on the product's risk level. For a simple product like a toy, a manufacturer can often self-declare conformity. That is absolutely not the case for TENS and EMS units, which are typically classified as Class IIa medical devices. The CE mark on these products must be accompanied by a four-digit number that identifies the Notified Body that performed the audit. This process is intense. The Notified Body examines everything from technical files and risk analysis to biocompatibility tests (ensuring the materials are safe for skin contact) and clinical data proving the device actually works as intended. If a supplier offers you a TENS device with a CE mark but no Notified Body number, you are looking at a non-compliant product that is illegal to sell in the EU.
Conclusion
Choose TENS for pain and EMS for muscle training. Vet your suppliers on quality systems, not just price. And always verify their regulatory compliance (CE and FDA) to protect your business.